甲醇电催化制氢膜电极的设计及性能优化毕业论文
2020-04-15 16:50:10
摘 要
能源促进了人类社会的发展,而如今,能源消耗与日俱增,其中以不可再生的化石能源为主。这种大幅度的依赖化石能源,不仅造成了严重的能源危机,还对环境产生了严重的污染。因此,发展可再生能源能源和提高能源的利用率成为目前人类必须要解决的重要问题。
燃料电池由于它具有接近零排放的优点,已经成为解决能源和环境问题的关键。而氢是燃料电池中的最佳燃料,但是要想使氢能最大效率的开发首先要解决制氢成本的问题。当前国内燃料电池上的制氢技术还不是很成熟,化学制氢仍是目前最主要的产氢手段。氢气的制取除了来自于化石燃料,还有其他方式。工业上的制氢技术主要采用水解制氢和甲醇制氢。其中水解制氢,由于其能耗较大,并不适合大规模使用。相比较水解制氢而言,电解甲醇只需要较低的电压,能耗更低。
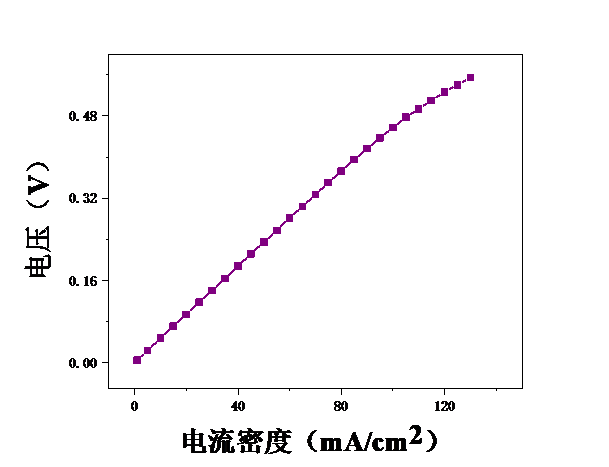
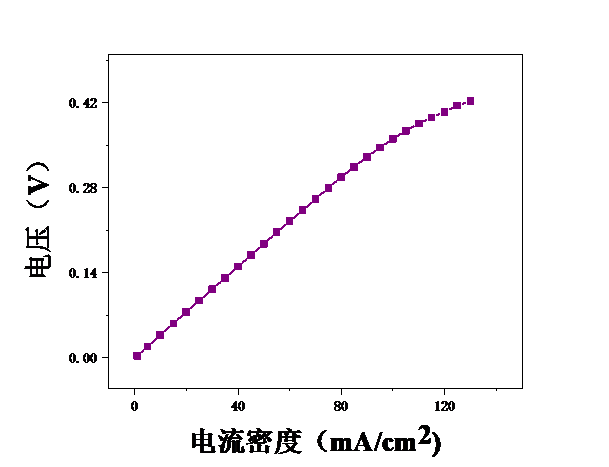
本论文想要研究甲醇电催化制氢技术,利用燃料电池来进行电解。首先,控制温度恒定在25℃,分别测试甲醇浓度为2mol/L、2.5mol/L、3mol/L、3.5mol/L、4mol/L、4.5mol/L、5mol/L时的电压,初步探究了甲醇浓度对膜电极性能的影响。结果表明,当甲醇浓度为4mol/L时,膜电极的性能最优。但在较高电流密度时,可能会出现极化现象。其次,保持甲醇浓度为5mol/L,分别测试温度为25℃、40℃、55℃、70℃时的电压实验中探究了测试温度对膜电极性能的影响。结果表明:随着温度的升高,甲醇的起始电接电压不断下降。
关键词:催化制氢 甲醇电催化氧化 燃料电池
Design and performance optimization of methanol electrocatalytic hydrogen membrane electrode
Abstract
Energy has promoted the development of human society. During this time, energy consumption grows day by day, besides where non-renewable fossil energy is the main one. Use fossil fuels caused a severe energy crisis, and also caused serious environmental pollution. energy energy and Renewable efficiency has become an important issue to be solved.
A fuel cell has been used to solve the questions of energy and environment owing to it is almost leaky.Hydrogen is the best fuel for fuel cells, but the cost of hydrogen production needs to be addressed before the most efficient hydrogen can be developed.Currently, hydrogen production technology in domestic fuel cells is not very mature, and chemical hydrogen production is still the main means of hydrogen production at present.Hydrogen can be made in other ways than from fossil fuels.Hydrogen production technology in industry mainly USES hydrolysis and methanol to produce hydrogen.Hydrogen hydrolysis is not suitable for large-scale use because of its high energy consumption.Compared with hydrogen production by hydrolysis, methanol electrolysis only requires lower voltage and energy consumption.
In this thesis, methanol electrocatalytic hydrogen production technology is studied, and fuel cell is used for electrolysis.At first, the control temperature was kept at 25℃, and the voltage of methanol concentrations of 2mol/L, 2.5mol/L, 3mol/L, 3.5mol/L, 4mol/L, 4.5mol/L and 5mol/L was tested, respectively, to preliminarily explore the influence of methanol concentration on the performance of membrane electrode.The results show that when the methanol concentration is 4mol/L,the membrane electrode has the best performance.However, polarization may occur at higher current densities.Secondly, the methanol concentration was maintained at 5mol/L, and the influence of the test temperature on the performance of the membrane electrode was explored in the voltage experiment when the test temperature was 25℃, 40℃, 55℃ and 70℃ respectively.The results show that the initial voltage of methanol decreases with the increase of temperature.
Key words: Catalytic hydrogen production;Electrocatalytic oxidation of methanol;fuel cell
目 录
摘要 I
Abstract II
第一章 绪论 4
1.1 引言 4
1.2发展氢能的必要性 5
1.3我国现阶段的制氢方法 6
1.4甲醇制氢的优势以及应用方式 9
1.5本论文的研究内容、目的及意义 9
1.5.1研究内容 9
1.5.2研究目的 9
1.5.3研究意义 10
第二章 实验设备与方法 10
2.1 实验试剂 10
2.2实验器材 11
2.3膜电极的制备 11
2.3.1.Nafion的预处理 11
2.3.2气体扩散层的制备 12
2.3.3催化层的制备 12
2.4单电池的组装 12
2.5质子膜电解甲醇性能测试 13
2.6质子膜电解甲醇的反应动力学 14
第三章 实验结果及分析讨论 16
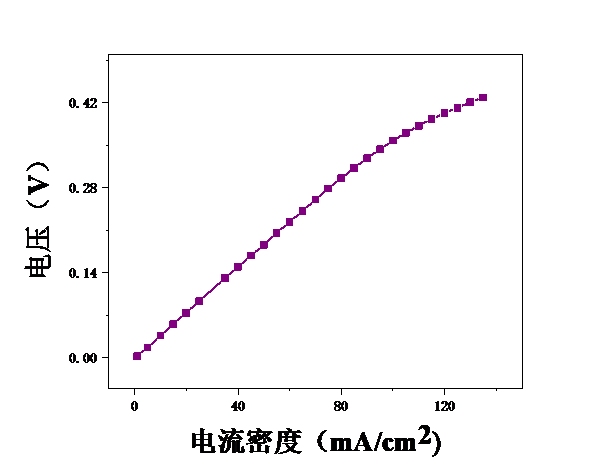
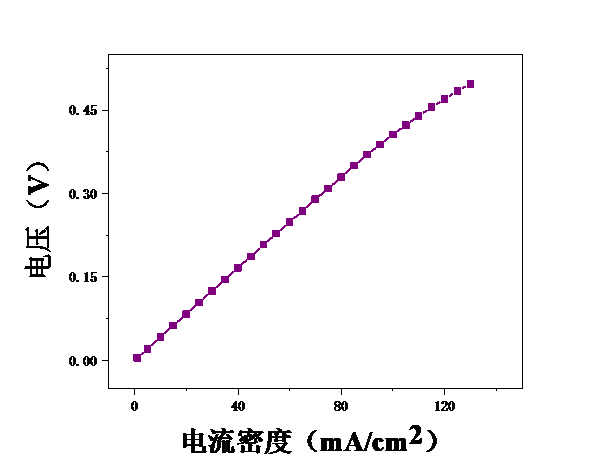
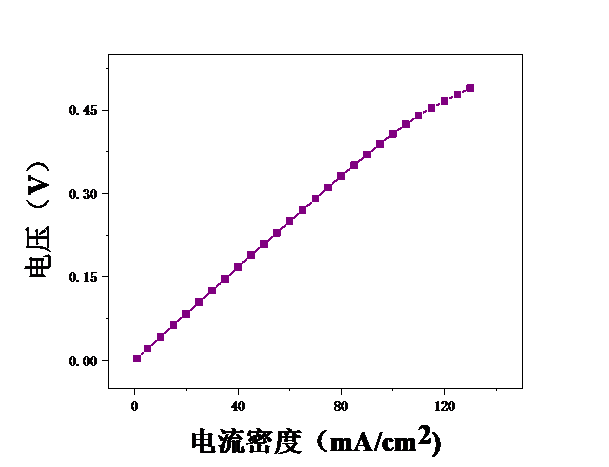
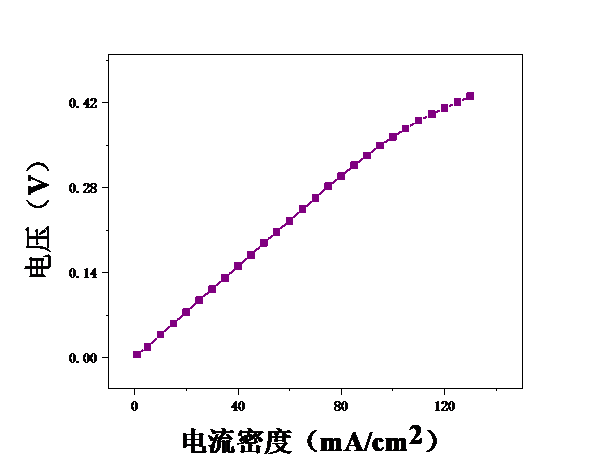
3.1甲醇浓度对膜电极性能的影响 16
3.2测试温度对膜电极的影响 19
第四章 总结与展望 21
4.1总结 21
4.2展望 21
参考文献 22
致谢 28
第一章 绪论
1.1 引言
现阶段,世界上面临着两大不容忽视的挑战:能源的快速消耗和环境的不断恶化。伴随着当代社会的经济快速发展,我们不可避免需要采用大量化石燃料来供能以及满足人们的日常所需的各项活动。煤炭、石油等不可再生能源不断的减少,人类社会迟早有一天会面临着传统化石能源枯竭的问题。与此同时,地球上的环境也变得不断恶劣起来。这两大挑战已经成为制约各国经济持续发展的因素。因此,寻找和发展可再生新能源对于当今社会的可持续发展更有着不可估量的价值和意义。不仅是中国还是全世界范围类的国家,都越来越重视能源和环境两大挑战,发展新能源也被推上日程,成为各个国家政府大力推广的对象。
以上是毕业论文大纲或资料介绍,该课题完整毕业论文、开题报告、任务书、程序设计、图纸设计等资料请添加微信获取,微信号:bysjorg。
相关图片展示:

您可能感兴趣的文章
- 单分散胶体CdSe和CdSe/CdS核/壳纳米晶体的两步合成策略外文翻译资料
- 沥青及沥青混合料产生的沥青烟的实验室评价外文翻译资料
- 溶胶凝胶法制备二氧化硅-氧化锆抗碱涂层外文翻译资料
- InAs/GaAs量子点尺寸对能带结构影响研究毕业论文
- InAs/GaAs量子点垒层对能带结构影响研究毕业论文
- 应用于3500K热辐射源的热光伏电池研究毕业论文
- 高(010)活性晶面暴露的BiVO4晶体的合成、改性及其光催化性能研究毕业论文
- 多孔氮化碳(g-C3N4)的合成、改性及其光催化性能研究毕业论文
- C/Cu2ZnSnS4(CZTS)复合材料制备及其光催化性能研究毕业论文
- Bi2MoO6/Cu2ZnSnS4(CZTS)复合材料制备及其光催化性能研究毕业论文




