多种油脂肪乳注射液(C6-24)全身主动过敏比较试验研究毕业论文
2020-07-05 17:25:36
摘 要
目的:使用豚鼠全身性活性过敏试验来测试多种油脂肪乳注射液(C6-24)的过敏反应。
方法:豚鼠36只,平分六组,牝牡参半。给予敏化3次,第二天给予试验产品高,低剂量,高剂量和低剂量对照,氯化钠注射液(0.9%)和卵白蛋白。最后一次注射后的两星期,每组动物(♀2只/♂1只)给高,低剂量的测试样品,高和低剂量对照,以及0.9%氯化钠液和卵白来进击,在最后一次注射后的三星期各组余下动物(♀1只/♂2只)给试验品高剂量和低剂量,高,低剂量对照,以及氯化钠液(0.9%)及卵白蛋白。全部激起的动物在激起打针到打针后约3小时具体查看并记实过敏反应环境、症状的起止时候。
结果:本尝试阴性对比组动物在2次激起打针后均未见较着的过敏反应症状。阳性对照组豚鼠表现出过敏症状,如在2次打针后都有呈现出紧张的不安宁、躁动、跳跃、步态不稳、灭亡等相关的一些症状。
关键词:多种油脂肪乳注射液(C6-24) 豚鼠 过敏反应
Comparative study on systemic active hypersensitivity of multiple oil fat emulsions (C6-24)
Abstract
Objective: to detect the potential anaphylaxis of a variety of oil Fat Emu Ision Injection (C6-24) by active allergy test in guinea pigs, and to provide reference for clinical trials.
Methods: 36 healthy guinea pigs, 6 males and a half females, were taken. The sensitized drug was given 3 times, and the high and low doses of the trial products, the high and low doses of the control products, the 0.9% Sodium Chloride Injection and the ovalbumin were given each other day by intraperitoneal injection.At the last fourteenth days after the last injection, 14 days after the last injection, each group of animals (♀ 2 / ♂ 1) gave high and low doses to the test products, 0.9% Sodium Chloride Injection and ovalbumin, and twenty-first days after the last injection, the remaining animals (♀ 1 / ♂ 2) were given high doses of the test. Low dose, high dose and low dose of reference substance, 0.9% Sodium Chloride Injection and ovalbumin attack. All animals were observed and recorded for 3 hours after the injection, and the onset and duration of the symptoms were recorded and recorded.
Results: in the negative control group, no obvious allergic reaction was observed after the 2 injection. In the positive control group, there were severe symptoms of restlessness, restlessness, gait instability, jumping and death after 2 injection.
Key words: A variety of oil fat emulsion injection(C6-24); Guinea pig; Allergic Reaction
目录
摘要 I
Abstract II
第一章 绪 论 1
1.1多种脂肪乳注射液(C6-24)相关知识 1
1.2药物相互作用 1
1.3药理毒理 1
1.4药代动力学 1
1.5临床使用注意事项 1
1.6豚鼠的过敏性实验 2
1.6.1全身主动过敏实验相关知识 3
1.6.2豚鼠的全身主动过敏性试验 3
1.7总结 4
第二章 多种脂肪乳注射液(C6-24)全身主动过敏实验研究 5
2.1研究依据 5
2.1.1 GLP法规依据 5
2.1.2质量保证 5
2.2试验日程 5
2.3实验仪器与材料 5
2.3.1、主要试验仪器 6
2.3.2供试品和对照品 6
2.3.3 实验动物 6
2.3.4实验系统选择理由 7
2.4 实验动物饲养管理 7
2.4.1饲养环境 7
2.4.2饲料 8
2.4.3饮用水 9
2.4.4动物检疫与适应 9
2.5 剂量设计 9
2.5.1给药剂量设计及选择理由 9
2.5.2供试品配制方式 9
2.5.3配制地点 10
2.5.4 剩余药液处理 10
2.5.5供试品和对照品给药数据 10
2.5.6给药方式选择的理由 11
2.6 实验方法与结果判定 11
2.6.1动物分组及实验剂量设计 11
2.6.2实验方法 11
2.6.3结果判定 12
第三章 结果与讨论 13
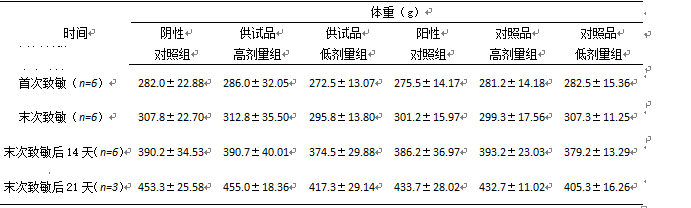
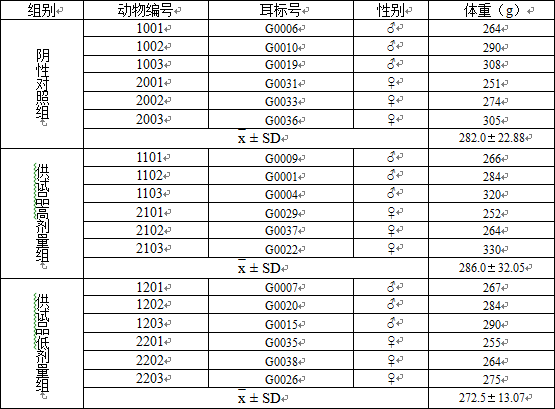
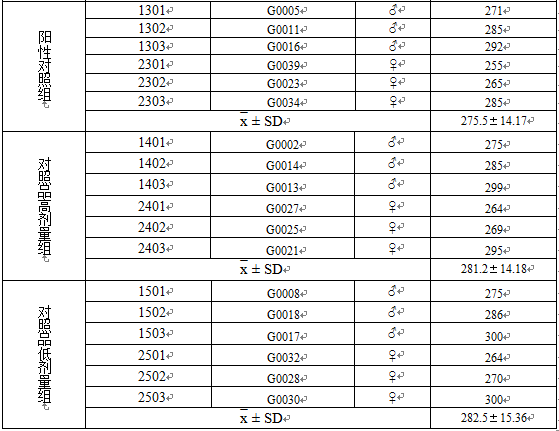
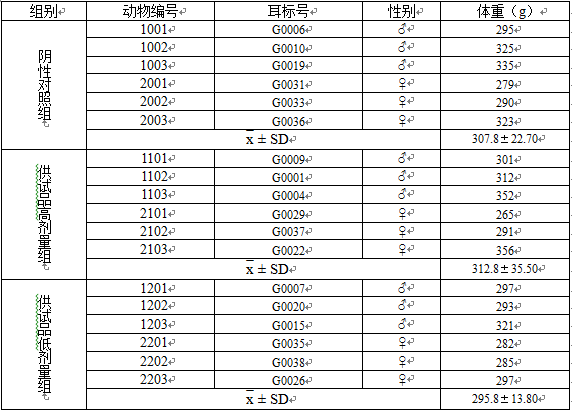
3.1称重数据 13
3.2实验结果 18
3.3实验小结 20
参考文献 21
致谢 23
第一章 绪 论
1.1多种脂肪乳注射液(C6-24)相关知识
通用名称:多种脂肪乳注射液(C6-24),英文名称:Multi-oil Fat Emulsion Injection(C6-24).
性状:白色乳状液体
适应症:用于肠外营养[1],为经口/肠道摄取营养不能、不足或有禁忌时的患者提供能量、必须脂肪酸和ω-3脂肪酸[2]。
1.2药物相互作用
相关图片展示: