达托霉素注射剂的稳定性研究毕业论文
2020-06-19 21:45:59
摘 要
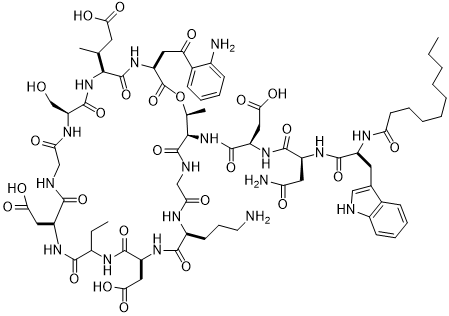
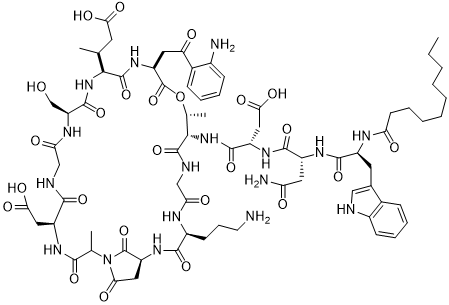
达托霉素在体外可以抗大多数的革兰氏阳性菌(G )。比如糖肽类敏感的金葡菌(GISA)、耐万古霉素的肠球菌(VRE)、耐甲氧西林的金葡菌(MRSA)和耐青霉素的肺炎链球菌(PRSP)等耐药致病菌。目前这些耐药菌在临床上可以选择的抗生素非常少,而达托霉素用于临床有明显的优势,它在临床中耐药菌株的出现率非常的低,应用前景是非常广阔的。所以研究达托霉素在临床中有着极其重要的意义。一般在临床上,达托霉素主要用来治疗复杂性的皮肤感染或者皮肤的软组织感染以及感染性内膜炎。但达托霉素对温度、pH值比较敏感。本课题主要研究达托霉素注射剂的稳定性。
本课题是由江苏奥赛康药业提供的注射用达托霉素。依据达托霉素的理化性质,通过文献调研,以达托霉素的有关物质等作为研究的主要考察指标对它的稳定性进行考察。本课题从含量和有关物质等项目为出发点对注射用达托霉素进行了稳定性研究试验。包括了注射用达托霉素的影响因素试验、注射用达托霉素的加速试验、注射用达托霉素的长期试验和注射用达托霉素的配伍稳定性试验。
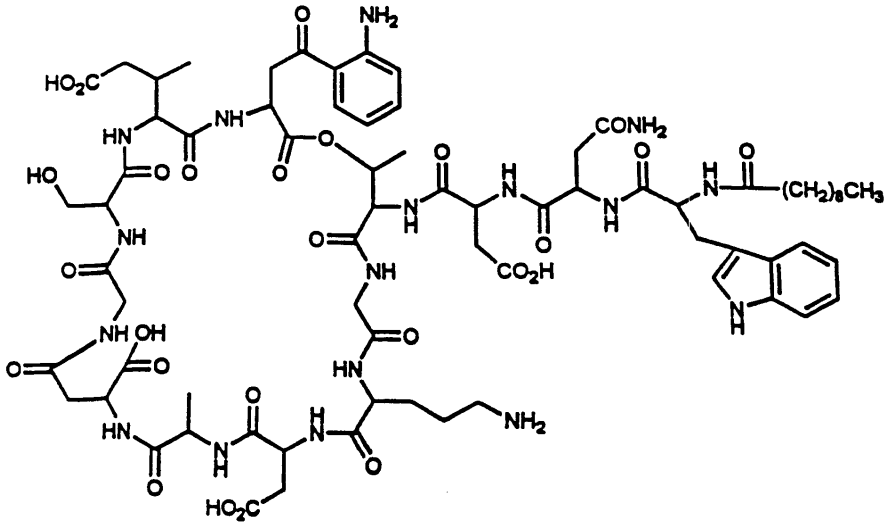
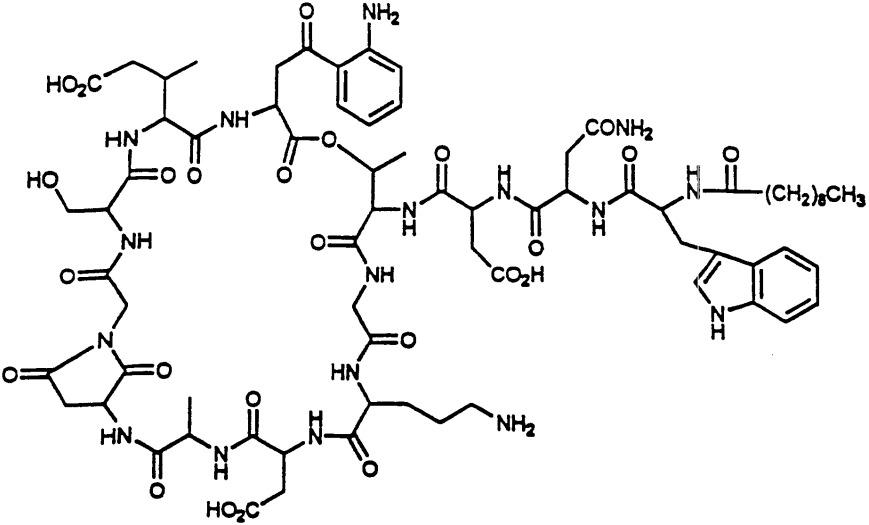
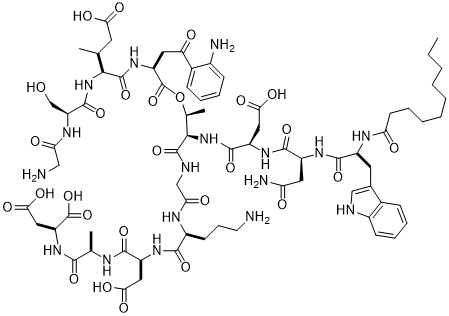
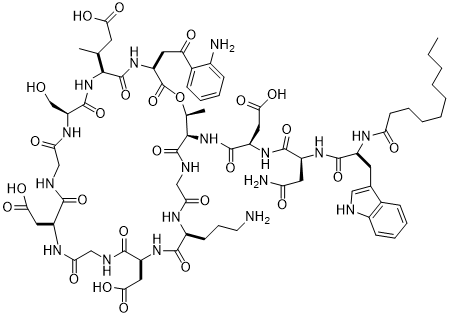
我们参照《中国药典》2015年版二部附录I A中对注射剂的要求以及对其原料的质量标准,我们对注射用达托霉素进行了酸度、有关物质检查、水分及含量测定等工作来对注射药达托霉素的质量标准进行研究。达托霉素对温度、pH都比较敏感。主要降解产物有内酯水解物、β-异构体、脱水达托霉素、杂质1、杂质2、杂质3、杂质4。可以根据本品检测出得主要强制降解物的紫外吸收情况,确定检测波长是214nm。最终确定了达托霉素有关物质的检测标准:内酯水解物(RT约为0.73)不得超过1.5%,脱水达托霉素(RT约为1.33)不得超过3.5 %,β异构体(RT约为0.86)不得超过2.0%,杂质1(RT约为0.58)不得超过0.5%,杂质2(RT约为0.90)不得超过1.5%,杂质3(RT约为1.15)不得超过1.0%,杂质4(RT约为1.33)不得超过0.5%,其他的最大单杂不得超过0.15%,总杂不得超过8.0%;采用高效液相色谱法来测定本品含量,检测波长应为214nm,如果按无水物计,达托霉素(C72H101N17O26)含量应该为87.0%~103.0%;如果按平均装量计,含量应该为标示量的95.0%~115.0%。经验证,本方法灵敏、准确、可靠、重复性高、专属性强,可以有效控制产品的质量。
关键词: 注射用达托霉素 质量研究 稳定性研究
Study on the Stability of Daptomycin For Injection
ABSTRACT
Daptomycin in vitro can be broad-spectrum anti-Gram-positive bacteria. These bacteria include resistant bacteria, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, glycopeptide-sensitive Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae and so on. At present, the clinical resistance of these antibiotics can choose antibiotics rarely, and daptomycin for clinical obvious advantages, its clinical drug-resistant strains of the occurrence rate is very low, the application prospect is very broad. Therefore, the study of daptomycin has a very important clinical significance. Mainly used to treat CSSSI, infective endometritis. However, daptomycin is particularly sensitive to temperature and pH, and this study focuses on the stability of daptomycin injection.
The subject is provided by the Jiangsu Aosai Kang medicine for the Dithomycin for injection. According to the physical and chemical properties of daptomycin, through the literature research, to daptomycin related substances as the main indicators. The stability of daptomycin for injection was studied from the aspects of influencing factors, accelerated test, long-term test and compatibility stability test from the related substances and contents.
Quality standard study: In order to control the quality of daptomycin for injection, we according to the physical and chemical properties of daptomycin, reference to "ChP" 2015 edition of the two Appendix IA on the requirements of the injection and its raw material quality standards, The quality of daptomycin for injection was tested for acidity, related substances, moisture and content determination. Refer to the "ChP" 2015 edition of the two Appendix Ⅵ H check acidity; the use of HPLC, the use of gradient elution method of this goods related to the detection of substances; reference to "ChP" 2015 edition two Appendix Ⅷ M first Method to check moisture. Daptomycin is sensitive to temperature and pH. The main degradation products are dehydrated daptomycin, β-isomer, lactone hydrolyzate, impurity 1, impurity 2, impurity 3, impurity 4. Can be detected according to this product was the main forced degradation of the UV absorption, to determine the detection wavelength is 214nm. (RT approx. 0.73) shall not exceed 1.5% and dehydrated daptomycin (RT approx. 1.33) shall not exceed 3.5% and the beta isomer (RT is approximately 0.86) shall not exceed the target of the test. More than 2.0%, impurity 1 (RT approx. 0.58) shall not exceed 0.5%, impurity 2 (RT approx. 0.90) shall not exceed 1.5%, impurity 3 (RT approx. 1.15) shall not exceed 1.0%, impurity 4 (RT approx. ) Can not exceed 0.5%, the other largest single complex no more than 0.15%, the total miscellaneous should not exceed 8.0%; Determination of the content of the product by high performance liquid chromatography, detection wavelength 214nm, according to the anhydrous meter, containing daptomycin (C72H101N17O26 ) Should be 87.0% ~ 103.0%; according to the average installed capacity, containing daptomycin (C72H101N17O26) should be marked the amount of 95.0% ~ 115.0%. The method of verification, the product of the relevant substances to check the method and content determination method sensitive, accurate, reliable, high repeatability, specificity, and can effectively control the quality of the product.
KEYWORDS:Dithomycin for injection; Quality research; Stability study
目 录
摘 要 I
ABSTRACT III
第一章 文献综述 1
1.1 达托霉素国内外研究情况 1
1.2 达托霉素的基本情况 2
1.3达托霉素作用机制 3
1.4 立题依据及研究思路 3
第二章:试验仪器和检测方法 4
2.1质量标准 4
2.2实验仪器 5
2.3检测项目及方法 6
2.3.1有关物质的检测 6
2.3.2含量测定 10
2.3.3 其他检测项目 11
2.4 小结 12
第三章 注射用达托霉素的稳定性研究 14
3.1 影响因素试验 14
3.1.1 影响因素试验结果 14
3.1.2 影响因素试验结论 17
3.2 加速试验 17
3.2.1 加速试验结果 17
3.2.2 加速试验结论 24
3.3 长期试验 25
3.3.1 长期试验结果 25
3.3.2 长期试验结论 28
3.4 配伍稳定性试验 28
3.4.1 配伍稳定性结果 28
3.4.2 配伍稳定性结论 32
3.5 小结 32
3.5.1 影响因素试验结论 32
3.5.2 加速试验结论 33
3.5.3 长期试验结论 33
3.5.4 配伍稳定性试验结论 34
参考文献 35
第一章 文献综述
1.1 达托霉素国内外研究情况
20世纪70年代以来,致病菌的抗生素耐药性已经成为一个不断升级的问题。由G 菌比如对青霉素和红霉素产生耐药性的肺炎球菌、耐万古霉素的肠球菌、对甲氧西林产生耐药性的葡萄球菌引起的感染问题[1,2]愈发严重。这些对于抗生素已经产生耐药性的高致病菌的接连产生使得临床上急切需要一种新型的抗生素[3]。而达托霉素对于上述的高致病耐药菌有很好的杀灭效果,而且达托霉素用药方便,毒副作用小[4]。所以达托霉素的上市为临床医生提供了一种新的治疗方案,可以最好的替代有“病原菌的最后一道防线”之称的万古霉素[5];并且,达托霉素的作用机制和化学结构和已经有的所有其他类别的抗生素都不相同,达托霉素作为环脂肽类抗生素家族的第一个产品[6-8]是继噁唑烷酮类抗生素后应用到临床的第一种新结构类别抗生素。
目前在环脂肽类抗生素这个大家族中,只有达托霉素这一物质进入到了临床研究的阶段,通过药效学实验研究的结果,我们可以看出它可以广谱抗所有已经发现的的革兰氏阳性病原菌,并且对高致病菌如耐青霉素肺炎链球菌(PRSP)、凝固酶阴性葡萄球菌(CNS)、糖肽类敏感金葡菌(GISA)等临床上常见的病菌都有很好的疗效。此外,由于达托霉素的作用机制和其他各类抗菌药物不相同,所以至今并未有达托霉素交叉耐药菌的报道,其临床耐药菌株的出现几率非常低(lt;0.2)[9,10]。2015年其在全球所占的市场份额达到11亿美元[11-16]。
相关图片展示: