新型铑催化剂在合成托法替尼合成中的运用毕业论文
2020-04-25 19:45:43
摘 要
Tofacitinib是辉瑞公司开发的一种JAK抑制剂,可有效抑制JAK1和JAK3的活性,阻断多种炎性细胞因子的信号转导。研究表明,托法替尼对类风湿关节炎、溃疡性结肠炎、银屑病等多种炎症相关疾病有良好的治疗效应。
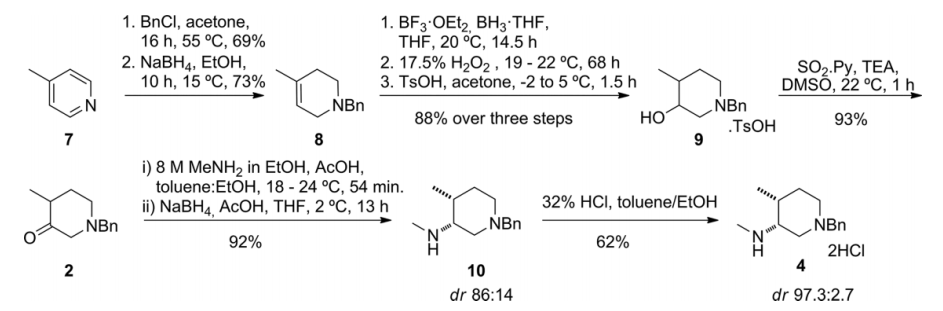
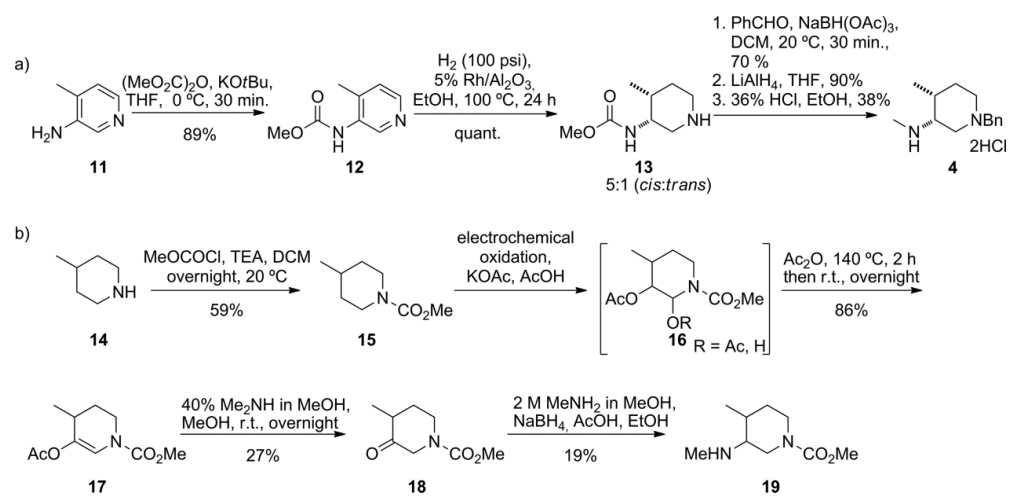
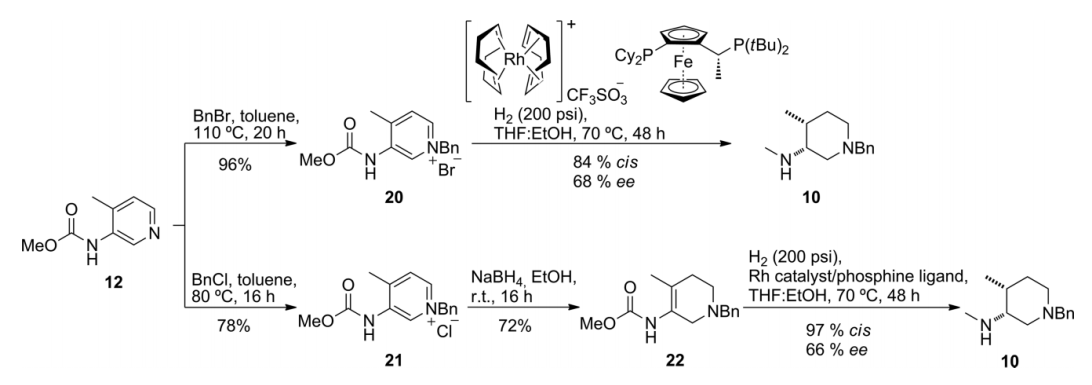
基于Tofacitinib在药学方面的重要性,学术界对它的合成有广泛的研究。其中,在合成关键中间体(3R,4R)-(1-苄基-4-甲基哌啶-3-基)甲胺上,如何获取单一的对映异构体是合成的重点也是难点。根据有关文献记载,在使用不同条件对Tofacitinib合成过程中的关键中间体进行合成的时候,使用手性铑络合物作为催化剂,能得到最高的顺/反式比。
手性过渡金属络合物催化一系列含有不饱和双键化合物与氢气分子发生不对称氢化反应,是合成手性化合物高效,高原子经济,环境友好的合成方法。
本论文利用铑催化剂这一优点,设想出一种优良的Tofacitinib的合成方案,利用新型铑催化剂在不对称氢化反应中表现出的高催化活性以及高对映选择性,在关键中间体的合成制备中,优化了反应条件,理论上可以实现更高的原子转化率。
综上所述,本论文结合前人及本课题组的相关工作经验,设计了一条药物Tofacitinib的合成线路,该路线具有很高的原子经济性和实际应用价值,但由于时间限制,未能完成整个合成步骤,对于铑在不对称氢化合成中的优势还有待进一步研究。
关键词:托法替尼 铑络合物 不对称氢化 对映异构体
Application of new ruthenium catalyst in the
synthesis of Tofacitinib
Abstract
Tofacitinib is a JAK inhibitor developed by Pfizer that effectively inhibits the activity of JAK1 and JAK3 and blocks the signal transduction of various inflammatory cytokines. Studies have shown that tofarinib has a good therapeutic effect on a variety of inflammation-related diseases such as rheumatoid arthritis, ulcerative colitis, and psoriasis.
Based on the importance of Tofacitinib in pharmacy, the academic community has extensive research on its synthesis. Among them, how to obtain a single enantiomer on the synthesis of the key intermediate (3R,4R)-(1-benzyl-4-methylpiperi
din-3-yl)methylamine is the key point of synthesis and difficulty. According to the literature, when the key intermediates in the synthesis of Tofacitinib are synthesized using different conditions, the highest cis/trans ratio can be obtained by using a chiral iridium complex as a catalyst.
The chiral transition metal complex catalyzes a series of asymmetric hydrogenation reactions of unsaturated double bond compounds with hydrogen molecules, and is a highly efficient, high atomic, environmentally friendly synthesis method for synthesizing chiral compounds.
In this paper, the advantages of ruthenium catalyst are used to envisage an excellent Tofacitinib synthesis scheme. The high catalytic activity and high enantioselectivity exhibited by the novel ruthenium catalyst in asymmetric hydrogenation reaction are synthesized in the preparation of key intermediates. In the process, the reaction conditions are optimized, and in theory, a higher atomic conversion rate can be achieved.
In summary, this paper combines the relevant work experience of the predecessors and the research group to design a synthetic line of Tofacitinib. This
route has high atomic economy and practical application value, but it cannot be completed due to time constraints. The overall synthetic step, the advantages of hydrazine in asymmetric hydrogenation synthesis, remains to be further studied.
Keywords: tofacitinib , ruthenium complex ,asymmetric hydrogenation ,
Enantiomer .
目 录
摘 要...........................................................................................................................I
Abstract..........................................................................................................................II
- 综述..................................................................................................................1
- 引言.................................................................................................................1
- 传统合成托法替尼的路线.............................................................................1
- (L-DTTA)手性拆分法....................................................................................2
- 逆合成法合成Tofacitinib关键中间体.........................................................3
- Tofacitinib逆合成分析..................................................................................5
1.5.1 哌啶部分的组装.................................................................................6
1.5.2 Tofacitinib的组装............................................................................12
1.6 本论文的研究工作.......................................................................................14
第二章 实验................................................................................................................16
2.1 实验部分.......................................................................................................16
2.1.1 实验仪器与试剂................................................................................16
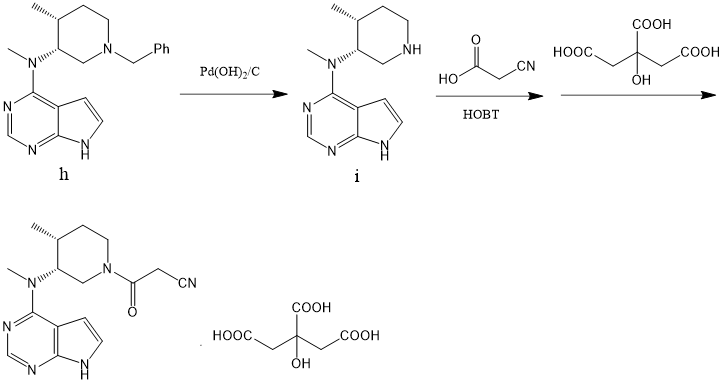
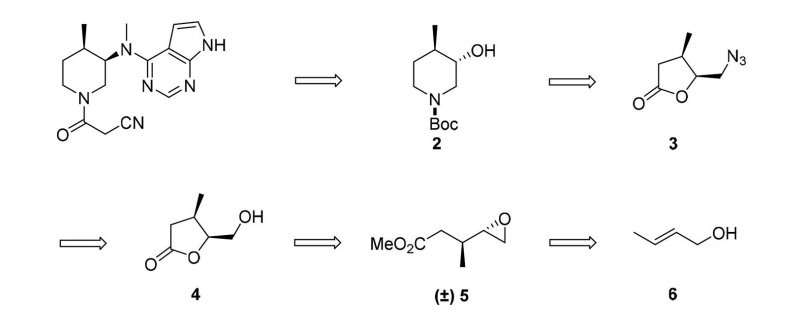
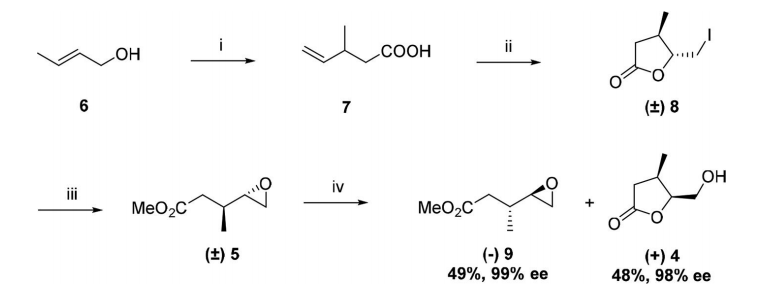
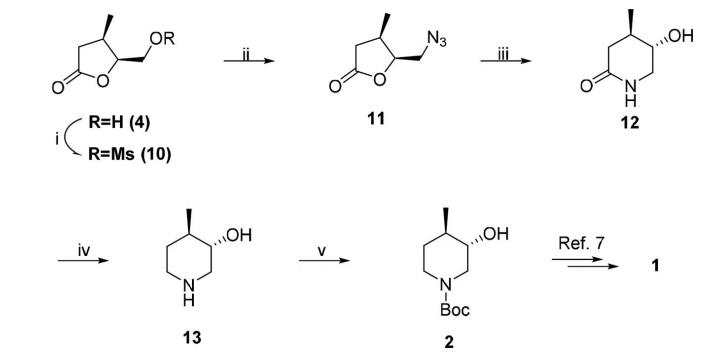
2.1.2 关键中间体(3R,4R)-(1-苄基-4-甲基哌啶-3-基)甲胺的制备..................................................................................................................................17
2.1.3 Tofacitinib的拼接.....................................................................
2.2 结果与讨论.........................................................................................
第三章 结论与展望......................................................................................
3.1 结论................................................................................................
3.2 展望.................................................................................................
参考文献...............................................................................................................
附 图.............................................................................................................
致 谢.........................................................................................................................
- 综述
- 引言
类风湿性关节炎是由免疫系统失调驱动的慢性自身免疫疾病,其影响世界上约1%的人群。到目前为止,传统的改善疾病的抗风湿药(DMARDs)或甲氨蝶呤(MXT)被用于治疗风湿性关节炎。由于获得的MXT和DMARD的治疗抗性/不良事件,正在寻找具有最小风险的新分子。 Janus蛋白酪氨酸激酶(JAK1,JAK2,JAK3和TYK2)在几种细胞因子的细胞内信号传导中起着起搏器的作用,并且已经在生物学进展,例如血细胞的生成和免疫炎症反应中发挥着重要作用。
枸橼酸托法替尼 ( tofacitinib citrate),化学名称为3-[(3R,4R)-4-甲基-3-[甲基-(7H-吡咯并[ 2,3-d]嘧啶-4-基)氨基]哌啶-1-基]-3-氧代丙腈枸橼酸盐,是由美国的辉瑞公司设计并经食药管理局(FDA)批准的JAK 3抑制剂,在大量的实际应用有显著效果,可运用于类风湿性关节炎,溃疡性结肠炎,和预防器官在移植过程中发生的排斥反应【1】。免疫抑制剂如环孢菌素A,他克莫司,霉酚酸酯和西罗莫司具有肾毒性,神经毒性,高脂血症和高血压等后果。 Tofacitinib被认为是克服这种后果的最佳选择【2】。
相关图片展示: