处理有机物污染废水藻类的筛选与调控研究毕业论文
2020-04-09 15:32:06
摘 要
四环素类抗生素是由放线菌产生的一类广谱抗生素,其价格低廉,抗菌谱广,是目前世界上使用最广泛、用量最大的抗生素种类之一。由于其用量大,吸收少,大部分以原药形式排出,容易在环境中积累。环境中残留的四环素类抗生素严重影响土壤生态环境和植物生长,对人类健康构成潜在危害。快速有效的清除环境中的抗生素残留问题已成为目前研究的热点。高级氧化技术去除水中四环素类抗生素存在着降解效率低、能耗高以及产生毒性更大的副产物等问题。吸附法存在吸附材料难以回收和脱附再利用的问题,导致成本较高。微藻能够利用CO2并生成氧气,有利于减少大气中二氧化碳含量,此外,从废水中回收微藻可以在污染物降解的同时实现资源化利用。目前,利用微生物降解抗生素的研究较少,且主要集中在细菌对抗生素的去除上。因此,开展微藻去除四环素的研究,可为四环素污染水体的生物修复提供理论依据,具有较大的理论意义和潜在的应用价值。
本研究以医院、制药厂等高浓度四环素污染废水为研究对象,通过研究藻类在四环素溶液中的生长行为,分析藻类降解四环素的可行性;通过研究藻类对四环素的降解效果,分析藻类对四环素的降解能力;通过对藻类降解四环素的调控,提出增强藻类降解四环素效果的方法。论文主要研究结果如下:
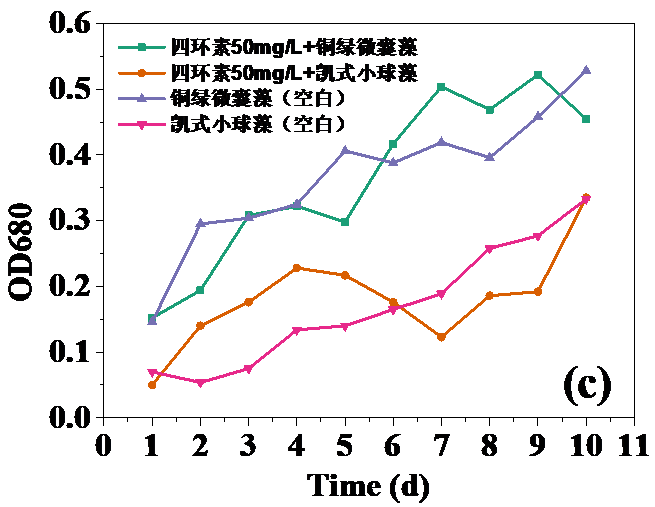
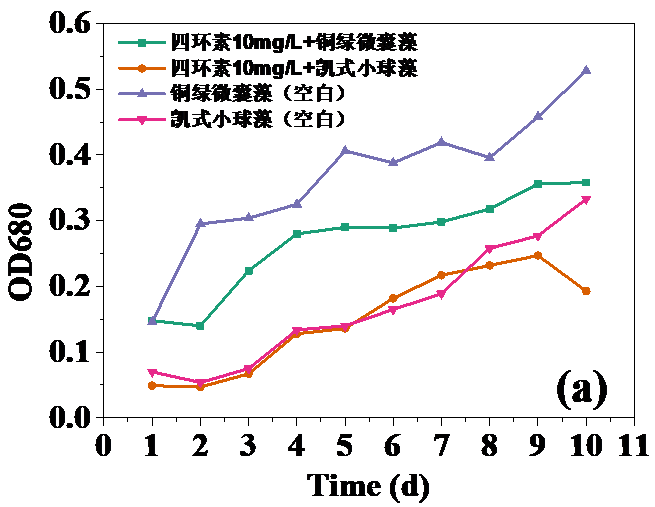
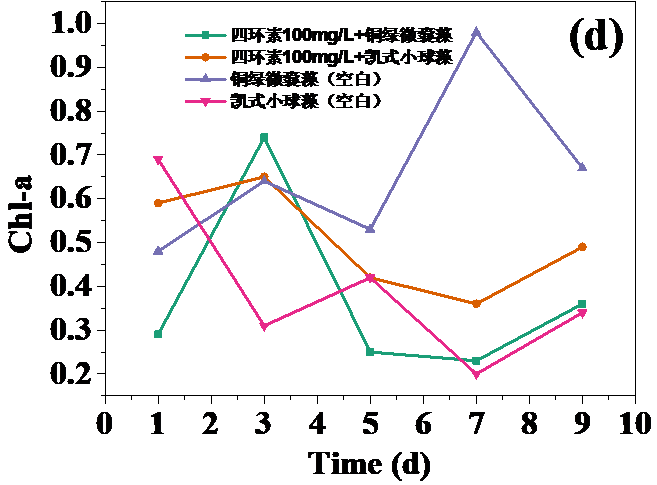
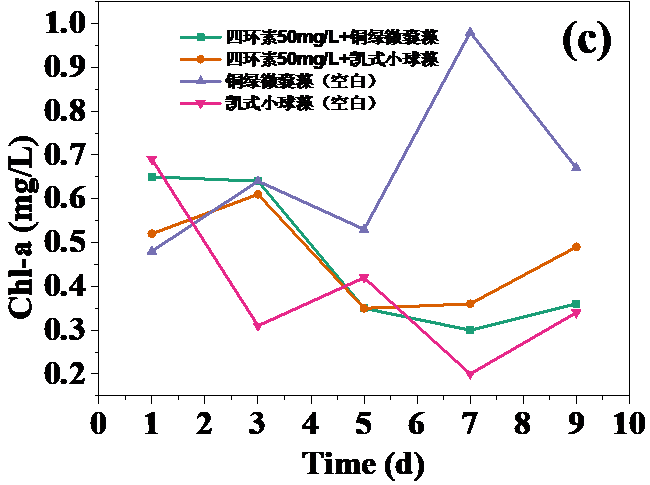
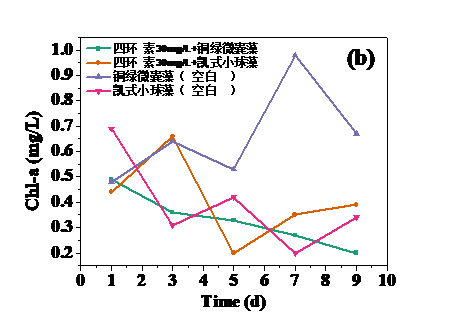
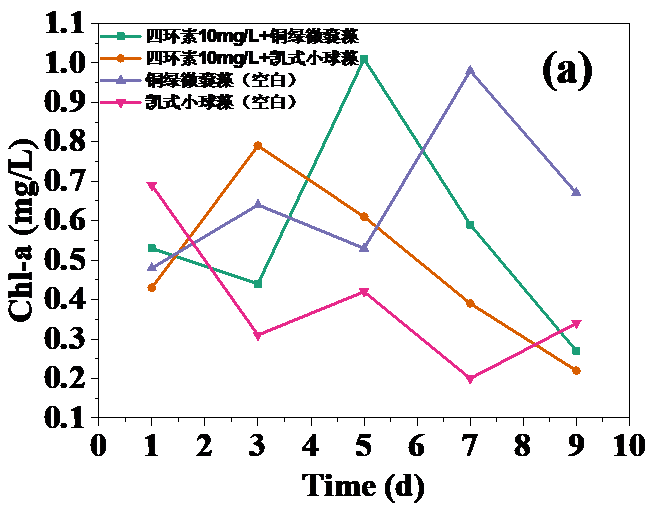
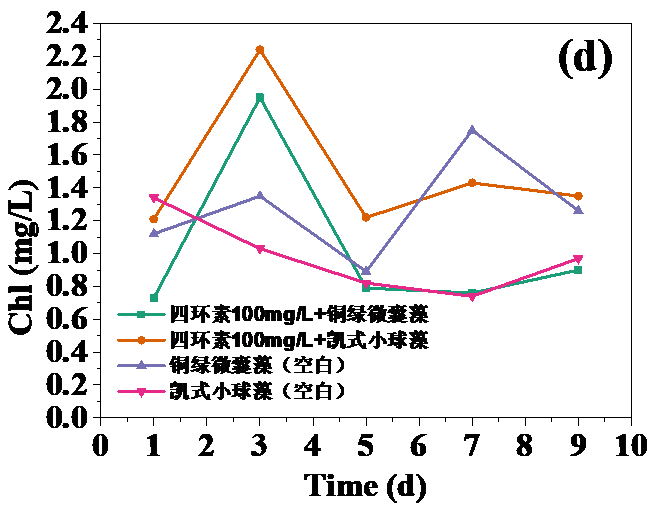
四环素浓度为10 mg/L和30 mg/L时,对藻类的生长起抑制作用,在实验第10天,对铜绿微囊藻生物量的抑制率分别为32.20%和24.62%,而对凯式拟小球藻生物量的抑制率分别为42.04%和30.93%。凯式拟小球藻的生长抑制率更高,说明凯式拟小球藻对四环素的毒性作用更为敏感。高浓度(50 mg/L和100 mg/L)明显促进了藻类的生长。在实验第10天,铜绿微囊藻生物量的增长率分别为13.83%和14.96%,而凯式拟小球藻生物量的增长率分别为0.60%和10.21%。推测四环素促进藻类生长的原因有:一定浓度的四环素首先使微藻产生应激反应,引起藻类生化特性的改变,提高藻类光合活性;四环素提高了共代谢中关键酶的浓度和活性;四环素去除了影响藻类生长的杂菌,从而促进了藻类的生长。当藻类刚暴露于四环素时,四环素能诱导藻类的应激反应,从而使其总叶绿素含量和叶绿素a含量的升高。随着与四环素接触时间增强,其对藻类的光合作用的影响逐渐体现,导致总叶绿素含量和叶绿素a含量降低。在实验后期,总叶绿素含量和叶绿素a含量逐渐升高,推测原因为藻类对四环素的毒性产生适应。
与对照组相比,藻类促进了四环素的降解,铜绿微囊藻对10 mg/L、30 mg/L、50 mg/L和100 mg/L的四环素的最终去除率分别为62.33%、76.14%、76.58%和89.37%;凯式拟小球藻对10 mg/L、30 mg/L、50 mg/L和100 mg/L的四环素的最终去除率分别为59.77%、72.60%、81.33%和87.65%。藻类在短期内能快速去除四环素,因为藻类对四环素的去除包括快速吸附,然后是缓慢的生物降解,生物吸附是藻类去除四环素的主要机制。藻类可通过生长代谢和共代谢降解四环素,Ⅰ相和Ⅱ相酶在四环素的生物降解中起着重要的作用。
MgSO4和Fe3O4调控下,铜绿微囊藻在四环素废水中的生长受到抑制,表明MgSO4和Fe3O4对微藻的毒性作用。在实验前期(前三天), TiO2促进了铜绿微囊藻在四环素溶液中的生长,原因为TiO2与四环素螯合,减弱了四环素对藻类的毒性作用,在实验第10天,TiO2表现出对藻类生长的抑制作用,与TiO2促进藻细胞内ROS的产生,从而影响藻类光合作用和藻类生长有关。而ZnCl2、BaCl2和MnCl2明显抑制了铜绿微囊藻的生长,使其生物量显著下降。MgSO4、BaCl2、ZnCl2和MnCl2能促进四环素的水解,从而促进四环素的去除。加入BaCl2和ZnCl2对四环素降解的促进作用最显著,在实验的第10天分别使四环素的去除率达到93.92%和94.66%,MgSO4和MnCl2使四环素的十日去除率分别达到89.00%和89.03%。TiO2和Fe3O4能光催化氧化四环素,在实验前三天明显促进了四环素的降解,但在实验第10天使四环素的含量升高,因为藻类吸附的四环素被重新释放到水体。TiO2还能诱导铜绿微囊藻以四环素为碳源进行代谢,以及吸附污染物,并运载其进入细胞内,从而促进藻类对四环素的去除。
关键词:四环素废水处理;废水养藻;藻资源化利用;生物调控。
Abstract
Tetracycline antibiotics (TCs) are broad-spectrum antibiotics with low price which was produced by actinomycetes. It is one of the largest types of antibiotic and widely used in the world. The TCs residues in the environment may lead to ecological risk and potential toxic effects due to its large dosage and most of them are discharged in the form of the parent compounds. The continuous accumulation of TCs residues in water poses a great threat to ecosystems and thus attracts extensive attention recently. TCs can be efficiently mineralize via several advanced oxidation processes (AOPs), including photocatalysis, ozonation, and Fenton chemistry. However, AOPs require considerable energy to completely convert TCs into carbon dioxide, rather, its intermediate products may be more toxic. Mixotrophic microalgae have especially gained research interests due to their endogenous catabolic systems, heterotrophic capabilities, role in the fixation and turnover of carbon, and their recovered biomass to produce bioenergy. Therefore, investigating removal of TCs by microalgae, can lead to better estimation of TCs in the environment and has the potential to be utilized in designing engineering processes to remove TCs from water.
The main objective of this study was to evaluate the ecotoxicological effects of TCs on Microcystis aeruginosa and Parachlorella kessleri by biomass, pigment (total chlorophyll and chlorophyll a) change and to evaluate removal efficiency of TCs by the two microalgae strains. M. aeruginosawas selected for further experiment, the enhanced removal efficiency of TCs by metal ion and metal oxide was evaluated. The main results were as follows:
The final biomass of M. aeruginosa and P. kessleri was significantly inhibited, showing 32.20% and 24.62% inhibition of M. aeruginosa, 42.04% and 30.93% inhibition of P. kessleri at 10 and 30 mg/L TCs, respectively, compared to the control. The biomass inhibition of P. kessleri was higher, indicating M. aeruginosais more resistant to TCs. While an increase in the concentration of TCs (50 and 100 mg/L) showed a significant increase in the microalgal growth after 1o days of cultivation.The biomass grew by 13.83% and 14.96% of M. aeruginosa, and0.60% and 10.21% of P. kessleri at 50 and 100 mg/L TCs, respectively, compared to the control. TCs promoted microalgae growth for several reasons: Microalgae have protective mechanisms, such as changes in biochemical characteristics, therefore promoted the photosynthetic processes; the addition of TCs can promote the activities of non-specific catabolic enzymes that are responsible for the degradation of the growth substrate; TCs inhibits the growth of bacteria, thus promoting the growth of microalgae. The increased total chlorophyll and chlorophyll a content in microalgae can be explained by the fact that chlorophyll act as a protective agent to neutralize the accumulated ROSs in cells. A gradual reduction in photosynthetic pigments is observed afterwards, showing the toxicity of TCs to microalgae. Microalgae exhibited recovery capacity toward TCs, because of the total chlorophyll and chlorophyll a content increased with an increase in the cultivation period.
M. aeruginosa showed 62.33%, 76.14%, 76.58% and 89.37% removal, while P. kessleri showed 62.33%, 76.14%, 76.58% and 89.37% removal of 10 mg/L, 30 mg/L, 50 mg/L and 100 mg/L, respectively. Biosorption dominated TCs removal, and accounted for the fast removal of TCs at the earlier experimental. Overall, TCs was removed through a cometabolic mechanism and intracellular Biodegradation, in which phase I and phase II enzyme families play a important role.
In addition of MgSO4 and Fe3O4,the biomass of M. aeruginosain50 mg/L TCs decreased, indicating the toxic effects of MgSO4 and Fe3O4 on microalgae.TiO2 has the ability to bind strongly to tetracycline, it is therefore hypothesized in this study that the TiO2 reduce TCs toxicity, leading to the promotion of the growth of M. aeruginosa in 50 mg/L TCs on the 3th days.TiO2 inhibited M. aeruginosa growth on the 10th days, which can be due to a increased accumulation of ROSs in cells induced by TiO2.Association with divalent metals affect the photolysis rates of TCs, and therefore promoted TCs removal. TCs removal reached 93.92%, 94.66%, 89.00% and 89.03% after adding BaCl2, ZnCl2, MgSO4 and MnCl2, respectively. Photocatalytic oxidation of tetracycline by TiO2 and Fe3O4 promoted the degradation of tetracycline significantly on the 3th days. However, the content of tetracycline on the 10th day increased because the tetracycline adsorbed by the microalgae was released into the water again.
Keywords: tetracycline wastewater treatment; microalgae growth; resources; utilization; regulation.
目录
摘要 I
Abstract III
第一章 绪论 1
1.1 引言 1
1.2 四环素去除方法的国内外研究现状 2
1.2.1 光降解 2
1.2.2 氧化降解 2
1.2.3 吸附 3
1.2.4 微生物降解 3
1.3 研究内容、目的及意义 4
1.3.1 目的及意义 4
1.3.2 研究内容 4
第二章 实验材料和方法 5
2.1 实验材料和仪器 5
2.2 实验藻株和培养条件 5
2.3 分析方法 5
2.3.4 水样中四环素含量的测定 6
第三章 四环素对藻类生长的影响 7
3.1引言 7
3.2 四环素对藻类生长的影响 7
3.3 四环素对藻类叶绿素a含量的影响 8
3.4 四环素对总叶绿素含量的影响 9
3.5 实验结果分析 10
3.5.1 四环素对藻类生长的影响 10
3.5.2 四环素对藻类总叶绿素和叶绿素a含量的影响 12
3.6 小结 12
第四章 藻类对四环素的去除效果 14
4.1 引言 14
4.2 四环素的水解和光解 14
4.3 溶液pH的变化 15
4.4 溶液溶解氧的变化 15
4.5 藻类对四环素的去除效果 16
4.6 实验结果分析 17
4.6.1 溶液pH的变化 17
4.6.2 溶液溶解氧的变化 17
4.6.3 藻类对四环素的去除效果 17
4.6 小结 18
第五章 藻类处理四环素污染废水的调控 20
5.1 引言 20
5.2 金属离子及金属氧化物调控下藻类在四环素废水中的生长行为 20
5.3 金属离子及金属氧化物对藻类降解四环素效果的影响 21
以上是毕业论文大纲或资料介绍,该课题完整毕业论文、开题报告、任务书、程序设计、图纸设计等资料请添加微信获取,微信号:bysjorg。
相关图片展示: